Hair Multiplication
aka - hair cloning
Hair multiplication, also known as hair cloning, is a cutting-edge technique aimed at countering hair loss. This approach involves extracting healthy follicle cells or dermal papillae from areas of the scalp unaffected by baldness. These cells are then cultured and multiplied in a laboratory before being injected back into bald areas to promote hair regrowth. The technology is still in its early stages, with most groups demonstrating partial success at small scales and some companies working towards commercial development.
Shiseido
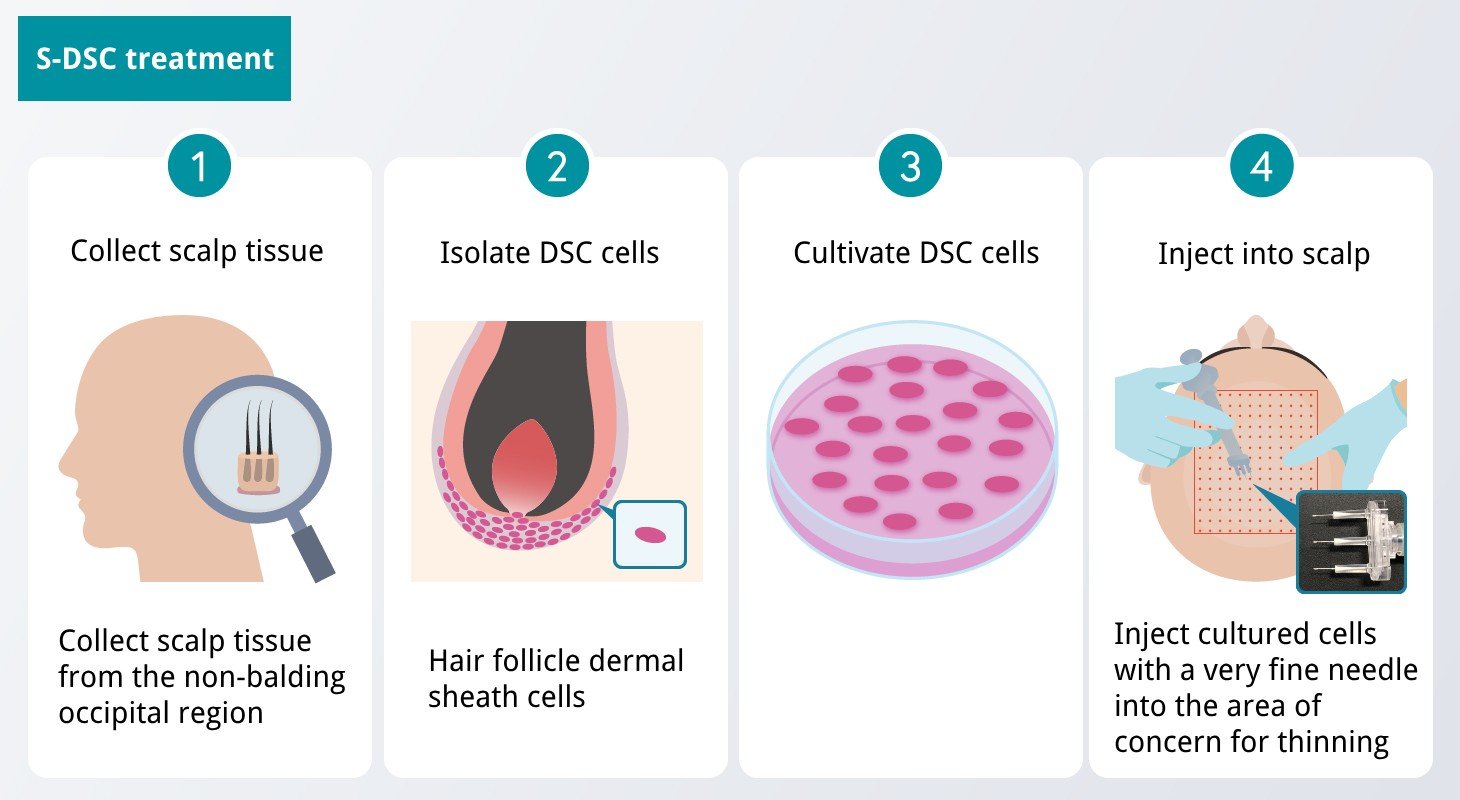
On June 28, 2024, Shiseido announced their Dermal Sheath Cup (DSC) treatment has arrived for patients in Japan, marking a significant milestone in the hair loss treatment landscape. Starting July 1, 2024, Associate Professor Shiro Niyama from Toho University Ohashi Hospital will offer this treatment using cultured autologous products now called S-DSC (the "S" is for Shiseido). More treatment facilities will follow, though no schedule has been published yet.
The treatment begins with a punch biopsy to isolate healthy hair follicles and extract DSC cells from the hair bulb. These cells are then multiplied in a growth medium and injected into the upper dermis of the scalp, where they once were expected to do everything from restore melanin pigment, sebaceous glands, and the growth of new hair fibers, but the actual results seem more modest. The cultured cells do demonstrably activate hair papilla cells, which in turn stimulate the division of hair matrix cells. This process leads to lower inflammation along with thicker and longer hair strands, effectively increasing perceptual density. Clinical studies have demonstrated notable improvements in hair appearance, particularly beneficial for individuals experiencing early-stage hair thinning, including women and those over 40.
One of the distinguishing features of S-DSC therapy is its safety profile. Since it employs the patient's own cells, the risk of rejection is minimal, making it a secure treatment option. Moreover, unlike traditional hair loss treatments that may be restricted by gender or require daily administration, S-DSC therapy is inclusive and requires only a few hospital visits. This reduced treatment frequency alleviates the burden on patients and enhances convenience.
History
In 2013, Vancouver-based RepliCel Life Sciences partnered with Shiseido, granting an exclusive license for their RCH-01 DSC cell hair multiplication technology in several Asian countries, for which Shiseido paid 400 million yen plus future royalties. RepliCel retained rights for the rest of the world. Shiseido continued trials along with some published research, and in 2021, RepliCel terminated the license agreement and initiated arbitration against Shiseido.
In 2023, Shiseido completed small-scale Phase 3 equivalent trials, achieving mildly positive outcomes. They published a few papers that year on identifying genes that helped cells move and integrate into hair follicles and showing their treatment increased hair thickness in men and women.
Other histories
In 2010, scientists at Berlin Technical University in Germany achieved a significant breakthrough by growing artificial hair follicles from animal stem cells. They created follicles thinner than normal but were optimistic about developing a method for cloning human hair from stem cells. Despite their confidence and the anticipation of a public release by 2015, the team encountered efficiency issues, cloning only one or two follicles per extracted hair instead of the required thousand for practical application.
During the same period, researchers at the University of Pennsylvania discovered that bald and non-bald scalps have the same number of stem cells. However, bald scalps showed a significant depletion in progenitor cells. This led to the hypothesis that hair loss might be due to the unsuccessful activation of these stem cells, rather than their absence. The team continued their investigations to find ways to convert regular stem cells into progenitor cells, potentially enabling natural hair regrowth on previously bald scalps.
Intercytex was one of the first companies to experiment with hair cloning, hoping to eliminate hereditary hair loss by growing new hair from a patient's own cells. In their Phase II trials, two-thirds of the male participants successfully grew new hair. However, Phase III trials did not meet expectations, and in 2008, Intercytex discontinued their hair cloning research due to both failed tests and financial instability. By 2010, Intercytex had gone out of business.
Aderans Research Institute (ARI) also pursued hair cloning with their "Ji Gami" process. This method involved extracting a small strip of scalp, breaking it down into individual follicular stem cells, culturing, multiplying, and injecting them back into bald areas. Although Phase II trials showed that the process could revitalize follicles and prevent further loss, it was not effective for multiplication. In July 2013, Aderans discontinued funding for its hair multiplication research.
In 2012, Durham University made progress by employing a new method for multiplying dermal papilla cells in a 3D system. Healthy dermal papillae were dissected and cultured, producing 3000 cells within 30 hours. These were injected into foreskin samples grafted on rats, and after six weeks, the cells formed brand-new hair follicles capable of growing hair. However, issues such as lack of pigmentation in some newly grown hair indicated that further studies were needed before human testing could commence.
In 2016, Japanese scientists at the Riken Centre for Developmental Biology, in collaboration with Organ Technologies and Kyocera Corporation, announced successful growth of human skin with hair in a lab. This breakthrough was achieved using induced pluripotent stem cells. OrganTech, formerly Organ Technologies, secured funding from Kobayashi Pharmaceutical in late 2022 and plans to transplant regenerated hair follicle primordia into humans by Q2 2024.
Yokohama National University researchers made history in October 2022 by successfully cloning fully-grown mouse hair follicles for the first time. This technology may take 5-10 years to be tested successfully in humans.
Stemson Therapeutics, based in San Diego, partnered with UCSD and achieved a significant milestone in July 2019 by growing hair follicles on a mouse using iPSC-derived epithelial and dermal cell therapy. The hair grew straight and aligned properly with a 3D-printed biodegradable shaft. Stemson plans to enter clinical trials in 2026.
In September 2023, TrichoSeeds, in collaboration with Rhoto Pharmaceutical, announced plans to begin clinical trials in 2024. Meanwhile, Epibiotech has developed an autologous dermal papilla cell scheduled for clinical trials at the end of 2023.
Research
20 / 1000+ results
research Application of Mesenchymal Stem Cells Derived from Bone Marrow and Umbilical Cord in Human Hair Multiplication
research Hair Multiplication With Dermal Papilla-Like Tissue Containing Human Dermal Papilla Cells
research Hedgehog Signaling Reprograms Hair Follicle Niche Fibroblasts to a Hyper-Activated State

research Hair Follicular Cell/Organ Culture in Tissue Engineering and Regenerative Medicine

research Donor Hair Follicle Preservation by Partial Follicular Unit Extraction: A Method to Optimize Hair Transplantation
research Advances in Hair Restoration: Wnt/Beta-Catenin Pathway and Stem Cell-Based Therapies

research Epidermal Stem Cells and Skin Tissue Engineering in Hair Follicle Regeneration

research Strategies to Enhance Epithelial-Mesenchymal Interactions for Human Hair Follicle Bioengineering

research Hair Physiology and Its Disorders

research Perspectives on Hair Evolution Based on Comparative Studies on Vertebrate Cornification

research Hair Follicles and Their Role in Skin Health

research Dental Derived Stem Cell Conditioned Media for Hair Growth Stimulation

research Follicular Cell Implantation: An Update on Hair Follicle Cloning

research Follicular Cell Implantation: An Emerging Cell Therapy for Hair Loss

research Protective Effects of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Against Dexamethasone-Induced Apoptotic Cell Death in Hair Follicles

research Evolution of Techniques in Hair Transplantation: A 12-Year Perspective

research Recreation of a Hair Follicle Regenerative Microenvironment: Successes and Pitfalls

research Hair Growth Promoting Effect of Dermal Papilla-Like Tissues from Canine Adipose-Derived Mesenchymal Stem Cells Through Vascular Endothelial Growth Factor

research Current Status of Hair Restoration Surgery