 September 2011 in “Cancer”
September 2011 in “Cancer” Men who start losing hair at 20 may have a higher chance of getting prostate cancer later.
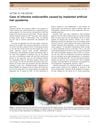
Adalimumab significantly improved Hidradenitis Suppurativa and quality of life in two patients.
[object Object] 20 citations,
January 2017 in “Epilepsia” Blocking neurosteroid production can lead to more seizures and faster epilepsy onset in rats.
4 citations,
May 2023 in “Cells” Baricitinib and its combination with lonafarnib improve fat cell formation in certain genetic disorders.
February 2024 in “Neurophotonics” Light therapy on the brain shows promise for treating brain diseases and improving brain function.
 1 citations,
June 2018 in “The Journal of Dermatology”
1 citations,
June 2018 in “The Journal of Dermatology” A man got heart infection from scalp infection caused by artificial hair implants.
 April 2023 in “Tikrit Journal of Pharmaceutical Sciences”
April 2023 in “Tikrit Journal of Pharmaceutical Sciences” Deferasirox effectively reduces iron overload in ß-thalassemia patients but may cause some manageable side effects.
 90 citations,
December 2007 in “Current Oncology”
90 citations,
December 2007 in “Current Oncology” Non-hormonal treatments should be used first for sexual dysfunction in postmenopausal breast cancer patients on aromatase inhibitors, with hormones as a second option.
 282 citations,
October 2006 in “The Journal of Clinical Endocrinology and Metabolism”
282 citations,
October 2006 in “The Journal of Clinical Endocrinology and Metabolism” The Endocrine Society advised against routine testosterone therapy for women, citing a need for more research on long-term safety and a clear definition of androgen deficiency.
 1265 citations,
October 2013 in “The Journal of Clinical Endocrinology and Metabolism”
1265 citations,
October 2013 in “The Journal of Clinical Endocrinology and Metabolism” The guideline suggests using specific criteria to diagnose PCOS, recommends various treatments for its symptoms, and advises screening for related health issues.
[object Object]  6 citations,
September 2005 in “Expert Opinion on Pharmacotherapy”
6 citations,
September 2005 in “Expert Opinion on Pharmacotherapy” Androgen therapy can help with symptoms like low libido in women, but more research is needed to understand its long-term safety and effects on health.
 23 citations,
January 2018 in “Elsevier eBooks”
23 citations,
January 2018 in “Elsevier eBooks” Nanoemulsions improve stability and delivery of active ingredients in cosmetics for skin and hair care.
 2 citations,
May 2021 in “Journal of pharmaceutical and biomedical analysis”
2 citations,
May 2021 in “Journal of pharmaceutical and biomedical analysis” A new method was developed to accurately detect and measure 47 different drug ingredients in various products.
 1 citations,
July 2015 in “Cambridge University Press eBooks”
1 citations,
July 2015 in “Cambridge University Press eBooks” Testosterone therapy can improve sexual function in women but long-term safety is unclear.
 1 citations,
November 2016 in “Congenital Anomalies”
1 citations,
November 2016 in “Congenital Anomalies” Get head MRI for babies with achondroplasia early, use free immunoglobulin light chains to detect certain neurodevelopmental disorders, and video calls work for speech therapy in patients with facial anomalies.
January 2025 in “International Journal of Pharmaceutics” The treatment showed significant hair regrowth in alopecia areata patients without side effects.
 28 citations,
November 2020 in “Journal of Controlled Release”
28 citations,
November 2020 in “Journal of Controlled Release” A new hair loss treatment uses tiny needles to deliver a drug-loaded lipid carrier, promoting hair growth more effectively than current treatments.
 21 citations,
January 2017 in “Skin Pharmacology and Physiology”
21 citations,
January 2017 in “Skin Pharmacology and Physiology” Caffeine-based liquid 0.2% is as effective as minoxidil 5% for treating male hair loss.
August 2024 in “International Journal of Molecular Sciences” Mesenchymal Stem Cell therapy shows promise for treating hair loss in Alopecia Areata.
 January 2025 in “Journal of Cosmetic Dermatology”
January 2025 in “Journal of Cosmetic Dermatology” Exosomes may help with hair growth and scar healing, but more research is needed.
Whale oil significantly promotes hair growth and may be a safe, effective alternative to minoxidil.
May 2024 in “Journal of Fungi” Tinea capitis in adults, especially postmenopausal Black women, needs prompt treatment with oral antifungals to avoid scarring.
 18 citations,
June 2010 in “Journal of Chromatography B”
18 citations,
June 2010 in “Journal of Chromatography B” New method measures finasteride in blood quickly and accurately.
 25 citations,
May 2020 in “Aesthetic Surgery Journal”
25 citations,
May 2020 in “Aesthetic Surgery Journal” The regenerative solution, tSVF, is a safe and effective treatment for various conditions like aged skin, scars, wounds, and more, but more research is needed to find the best way to use it.
 24 citations,
January 2019 in “Science China Life Sciences”
24 citations,
January 2019 in “Science China Life Sciences” Chitosan/LiCl composite scaffolds help heal deep skin wounds better.
17 citations,
January 2019 in “Journal of cutaneous medicine and surgery” JAK inhibitors show promise for treating hair loss in alopecia areata but need more clinical trials to confirm safety and effectiveness.
 2 citations,
January 2019
2 citations,
January 2019 The document concludes that autoimmune skin disorders are treated with corticosteroids and immunosuppressive drugs.
 1 citations,
June 2010 in “Expert Review of Dermatology”
1 citations,
June 2010 in “Expert Review of Dermatology” Covers common skin issues in kids, their diagnosis, treatment, and need for specialist care.
 59 citations,
March 2020 in “Journal of Biomedical Science”
59 citations,
March 2020 in “Journal of Biomedical Science” Understanding how hair follicle stem cells work can help find new ways to prevent hair loss and promote hair growth.
 January 2019 in “Research Square (Research Square)”
January 2019 in “Research Square (Research Square)” The trial will test if YH0618 granule prevents hair loss in breast cancer patients during chemotherapy.





















