 August 2019 in “Regenerative Medicine”
August 2019 in “Regenerative Medicine” In June 2019, the stem cell research field saw major progress, including new clinical trials, FDA approvals, and industry collaborations.
 2 citations,
October 1998 in “Family Practice”
2 citations,
October 1998 in “Family Practice” New oral treatment, finasteride, effectively and safely treats common hair loss.
 32 citations,
March 2020 in “Drug Design Development and Therapy”
32 citations,
March 2020 in “Drug Design Development and Therapy” Finasteride shows promise for female hair loss, but more research needed.
[object Object]  8 citations,
August 2020 in “Experimental dermatology”
8 citations,
August 2020 in “Experimental dermatology” PRP therapy for alopecia shows inconsistent results due to natural variability in growth factor secretion by platelets.
 July 2003 in “Journal of Cutaneous Medicine and Surgery”
July 2003 in “Journal of Cutaneous Medicine and Surgery” The document concludes that various treatments for skin conditions are effective, but some require further research, and certain factors like gender and lifestyle can influence disease outcomes.
 5 citations,
September 2015 in “PubMed”
5 citations,
September 2015 in “PubMed” New treatments for progressive hair loss in aging men are being developed, including targeted medical therapies, light therapy, plasma injections, and robotic hair transplantation.
 June 2018 in “Advances in Cosmetic Surgery”
June 2018 in “Advances in Cosmetic Surgery” Hair loss caused by genetics and hormones; more research needed for treatments.
 1 citations,
May 2014 in “Lipid Technology”
1 citations,
May 2014 in “Lipid Technology” New cleaning surfactants, biofuel production plans, dairy expansions, improved lipid testing methods, and various product launches and developments were reported in lipid technology.
 May 2019 in “Cytotherapy”
May 2019 in “Cytotherapy” Patients in Australia underwent costly, unproven stem cell treatments due to weak regulations and aggressive marketing.
 5 citations,
February 2012 in “Aesthetic Plastic Surgery”
5 citations,
February 2012 in “Aesthetic Plastic Surgery” A man developed skin cancer on his scalp after multiple artificial hair grafts.
 37 citations,
May 2003 in “Journal of Consumer Marketing”
37 citations,
May 2003 in “Journal of Consumer Marketing” The debate on direct-to-consumer pharmaceutical ads continues, with consumers finding them educational and doctors concerned about their impact on patient relationships and medication understanding.
 15 citations,
March 2020 in “Anais Brasileiros De Dermatologia”
15 citations,
March 2020 in “Anais Brasileiros De Dermatologia” Finasteride may cause lasting sexual, mental, and physical symptoms; use with caution.
 January 2024 in “Clinical, cosmetic and investigational dermatology”
January 2024 in “Clinical, cosmetic and investigational dermatology” Dermatologists should customize cosmetic treatments for dark-skinned patients to minimize risks and complications.
 January 2019 in “ARC journal of pharmaceutical sciences”
January 2019 in “ARC journal of pharmaceutical sciences” Acne can be managed with various treatments and requires psychological support due to its emotional impact.
5 citations,
March 2022 in “Frontiers in Cell and Developmental Biology” Colostrum-derived exosomes can promote hair growth and may be a promising treatment for hair loss.
17 citations,
November 2021 in “Journal of Cosmetic Dermatology” Combination therapies for androgenetic alopecia work best but can have significant side effects and costs.
 40 citations,
December 2019 in “Neurobiology of Stress”
40 citations,
December 2019 in “Neurobiology of Stress” Neuroactive steroids show promise for treating mental and neurological disorders by targeting GABA_A receptors.
 9 citations,
December 2020 in “Dermatologic Therapy”
9 citations,
December 2020 in “Dermatologic Therapy” Certain drugs are effective for skin conditions like psoriasis, vitiligo, and hair loss.
[object Object]  2 citations,
January 2019 in “Indian Dermatology Online Journal”
2 citations,
January 2019 in “Indian Dermatology Online Journal” The congress concluded that misuse of antifungal drugs in South Asia has led to widespread treatment failure, and new approaches and regional cooperation are needed.
 November 2020 in “Elsevier eBooks”
November 2020 in “Elsevier eBooks” Antiandrogens and androgen inhibitors like spironolactone, finasteride, and dutasteride can treat hair loss and skin conditions, but they have risks and side effects, including potential harm to pregnant women and risks of cancer and heart issues. Herbal remedies also have antiandrogenic effects but lack safety validation.
 January 2002 in “Journal of Toxicology-cutaneous and Ocular Toxicology”
January 2002 in “Journal of Toxicology-cutaneous and Ocular Toxicology” Botanical extracts are increasingly important in cosmetics and drugs for their effectiveness and safety, backed by traditional use and scientific evidence.
 3 citations,
July 2020 in “Frontiers in Cell and Developmental Biology”
3 citations,
July 2020 in “Frontiers in Cell and Developmental Biology” Vitexin Compound 1 may help reduce skin aging caused by UVA light.
18 citations,
September 2021 in “Journal of Neuroendocrinology” Neurosteroids can influence behavior by modulating brain inhibition, with potential for treating psychiatric disorders.
 May 2024 in “Journal of cosmetic dermatology”
May 2024 in “Journal of cosmetic dermatology” Heat-treated Limosilactobacillus fermentum with menthol, salicylic acid, and panthenol promotes hair growth and balances scalp microbiome in people with androgenetic alopecia.
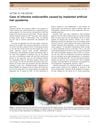 1 citations,
June 2018 in “The Journal of Dermatology”
1 citations,
June 2018 in “The Journal of Dermatology” A man got heart infection from scalp infection caused by artificial hair implants.
 March 2021 in “Egyptian Journal of Chemistry”
March 2021 in “Egyptian Journal of Chemistry” The herbal shampoo with Momordica charantia and Hibiscus Rosa-Sinensis is safe and promotes hair growth and scalp health.
 March 2007 in “The FASEB Journal”
March 2007 in “The FASEB Journal” Henna mixed with PPD can cause skin reactions, scarring, and a specific type of baldness, and needs more research to understand these effects.
 9 citations,
September 2016 in “Medical Clinics of North America”
9 citations,
September 2016 in “Medical Clinics of North America” Eating less and exercising more, with personalized diet plans and realistic goals, can lead to weight loss and better health, but more research is needed for long-term success.
 51 citations,
May 2013 in “The Journal of Steroid Biochemistry and Molecular Biology”
51 citations,
May 2013 in “The Journal of Steroid Biochemistry and Molecular Biology” Certain drugs that block specific enzymes can help treat prostate diseases.
 3 citations,
May 2018 in “InTech eBooks”
3 citations,
May 2018 in “InTech eBooks” Animal models, especially mice, are essential for advancing hair loss research and treatment.



























