Latanoprost
aka - Xalatan
- PhXA41
Latanoprost is a synthetic prostaglandin analog commonly prescribed for lowering intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Beyond its primary use, latanoprost has been explored for its potential in promoting hair growth due to its influence on hair follicle dynamics. The drug's mechanism of action in hair growth involves mimicking the effects of naturally occurring prostaglandins, which are known to play a role in the hair growth cycle, potentially by shifting hair follicles from the telogen (resting) phase to the anagen (growing) phase, thereby stimulating hair growth and increasing hair density.
Research into the effects of latanoprost on hair growth has yielded promising results. For instance, a pilot study on the topical application of latanoprost 0.1% showed an increase in hair density and pigmentation in individuals with androgenetic alopecia, suggesting that latanoprost may be beneficial for stimulating hair follicle activity. Furthermore, studies involving patients with eyelash alopecia have demonstrated the effectiveness of latanoprost in promoting eyelash regrowth, with a significant percentage of patients showing noticeable improvement without adverse effects.
Community sentiment regarding the use of latanoprost for hair growth is cautiously optimistic. Users have reported noticeable improvements in hair density and overall scalp health, especially when latanoprost is used in conjunction with other hair loss treatments such as minoxidil or finasteride. However, experiences vary, and some users emphasize the importance of managing expectations and acknowledging that latanoprost may not work for everyone.
While more extensive clinical trials are necessary to fully understand its efficacy and safety as a hair growth treatment, early research and positive community feedback highlight latanoprost's promise.
Research
20 / 456 results
research Hypertrichosis and Increased Pigmentation of Eyelashes and Adjacent Hair in the Region of the Ipsilateral Eyelids of Patients Treated with Unilateral Topical Latanoprost

research A Randomized Double-Blind Placebo-Controlled Pilot Study to Assess the Efficacy of a 24-Week Topical Treatment by Latanoprost 0.1% on Hair Growth and Pigmentation in Healthy Volunteers with Androgenetic Alopecia

research Lack of Efficacy of Topical Latanoprost and Bimatoprost Ophthalmic Solutions in Promoting Eyelash Growth in Patients with Alopecia Areata

research Latanoprost in the Treatment of Eyelash Alopecia in Alopecia Areata Universalis

research Hypertrichosis Induced by Latanoprost

research Lack of Efficacy of Topical Latanoprost in the Treatment of Eyebrow Alopecia Areata

research Effect of Latanoprost on Hair Growth in the Bald Scalp of the Stump-Tailed Macaque: A Pilot Study
research Latanoprost Loaded in Polymeric Nanocapsules for Effective Topical Treatment of Alopecia
research Lash Ptosis Caused by Latanoprost

research Cutaneous Latanoprost in the Treatment of Alopecia Areata

research Erosive Pustular Dermatosis of the Scalp Following Topical Latanoprost for Androgenetic Alopecia

research The Efficacy of Latanoprost in the Treatment of Alopecia Areata of Eyelashes and Eyebrows

research Efficacy of Topical Latanoprost Versus Minoxidil and Betamethasone Valerate on the Treatment of Alopecia Areata

research Latanoprostene Bunod Ophthalmic Solution 0.024% in the Treatment of Open-Angle Glaucoma: Design, Development, and Place in Therapy

research Nanostructured Lipid Carriers Loaded with Minoxidil and Latanoprost for Targeted Topical Therapy of Alopecia

research LC-MS Bioanalytical Method for Simultaneous Determination of Latanoprost and Minoxidil in the Skin
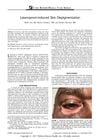
research Latanoprost-Induced Skin Depigmentation

research Repigmentation of Hair After Latanoprost Therapy
