 January 2024 in “American journal of clinical dermatology”
January 2024 in “American journal of clinical dermatology” Ritlecitinib is safe and well-tolerated for treating alopecia areata in patients aged 12 and older.
 2 citations
,
April 2023 in “JEADV. Journal of the European Academy of Dermatology and Venereology/Journal of the European Academy of Dermatology and Venereology”
2 citations
,
April 2023 in “JEADV. Journal of the European Academy of Dermatology and Venereology/Journal of the European Academy of Dermatology and Venereology” JAK-inhibitors for alopecia areata are generally safe with mostly mild side effects and a low rate of treatment withdrawal.
290 citations
,
August 2021 in “Clinical Reviews in Allergy & Immunology” JAK inhibitors show promise for treating alopecia areata, but more research is needed.
138 citations
,
March 2021 in “Journal of the American Academy of Dermatology” Ritlecitinib and brepocitinib effectively regrow hair in alopecia areata patients.
 20 citations
,
August 2018 in “Clinics in Dermatology”
20 citations
,
August 2018 in “Clinics in Dermatology” The conclusion is that understanding and addressing the psychological effects of alopecia areata is important for effective treatment.
 100 citations
,
July 2018 in “Journal of The American Academy of Dermatology”
100 citations
,
July 2018 in “Journal of The American Academy of Dermatology” People with alopecia areata often have other health issues like skin diseases, metabolic syndrome, stomach infections, lupus, anemia, thyroid problems, mental health issues, vitamin D deficiency, and hearing and eye problems.
 290 citations
,
December 2017 in “Journal of The American Academy of Dermatology”
290 citations
,
December 2017 in “Journal of The American Academy of Dermatology” Alopecia areata is an autoimmune condition causing hair loss, influenced by genetics, stress, and diet, and may be prevented by a high soy oil diet.
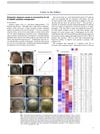 68 citations
,
November 2015 in “The Journal of Allergy and Clinical Immunology”
68 citations
,
November 2015 in “The Journal of Allergy and Clinical Immunology” Blocking IL-12/IL-23p40 helped reverse severe hair loss in patients.
 176 citations
,
August 2015 in “The journal of allergy and clinical immunology/Journal of allergy and clinical immunology/The journal of allergy and clinical immunology”
176 citations
,
August 2015 in “The journal of allergy and clinical immunology/Journal of allergy and clinical immunology/The journal of allergy and clinical immunology” Alopecia areata involves immune activation in the scalp, suggesting treatments targeting TH1, TH2, and IL-23 pathways.
21 citations
,
October 2007 in “Australasian Journal of Dermatology” A woman with hair loss regrew white hair after taking prednisolone.











