Review Article: Dermatological Complications of Immunosuppressive and Anti-TNF Therapy in Inflammatory Bowel Disease
September 2013
in “
Alimentary Pharmacology & Therapeutics
”

TLDR Immunosuppressive and anti-TNF therapies in IBD patients can increase the risk of skin cancer and cause various skin issues.
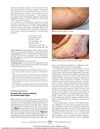
The 2013 review article examined the dermatological complications associated with immunosuppressive and anti-TNF therapy in patients with inflammatory bowel disease (IBD). It found that thiopurines like azathioprine and mercaptopurine significantly increased the risk of non-melanoma skin cancer (NMSC), especially in Caucasians over 65 years old. Anti-TNF therapy was associated with a 1% incidence of drug-induced lupus and up to a 3% incidence of psoriatic rash. The combination of thiopurines and anti-TNF agents further increased the risk of NMSC and lymphoma. The CESAME French nationwide observational cohort study, which included 19,486 IBD patients, identified both current and past thiopurine treatment as significant risk factors for NMSC. Methotrexate was linked to rashes and alopecia, and a 3-fold increased risk of malignant melanoma in rheumatology patients, though data in IBD patients were lacking. Calcineurin inhibitors were associated with cutaneous malignancies in long-term use. Up to a fifth of patients on anti-TNF therapy could experience dermatological side effects, including infections, psoriasis, and alopecia. The review emphasized the need for regular skin cancer screening, comprehensive dermatological examinations, and collaboration between gastroenterologists and dermatologists to manage these adverse events effectively.