TLDR Oral tofacitinib has a moderate success rate and is generally safe for treating hair loss in some patients.
In a retrospective study involving nine patients with alopecia areata (AA) or alopecia universalis (AU), aged between 13 and 33 years, the efficacy of oral tofacitinib (TOFA) was assessed after a minimum of 6 months of treatment. The study found that 44.4% of the patients did not respond to the treatment, 33.3% showed a moderate response, 11.1% had an intermediate response, and 11.1% experienced a complete response, resulting in an overall clinical response rate of 41.4%. Most of the responders had AA rather than AU. The adverse effects reported were mild, with two patients experiencing upper respiratory tract infections and one patient developing proteinuria. The study concluded that TOFA seems to have a reasonable clinical response rate and is well-tolerated, but emphasized the need for further randomized controlled trials to fully evaluate its efficacy, adverse effects, and the durability of the treatment for AA.
 17 citations
,
January 2019 in “Dermatologic Therapy”
17 citations
,
January 2019 in “Dermatologic Therapy” Tofacitinib is effective and safe for long-term treatment of severe alopecia areata, with many patients achieving complete hair regrowth.
 66 citations
,
December 2018 in “Dermatology”
66 citations
,
December 2018 in “Dermatology” Both ruxolitinib and tofacitinib are effective and safe for treating severe alopecia areata, but relapses are common.
 49 citations
,
March 2017 in “Journal of the American Academy of Dermatology”
49 citations
,
March 2017 in “Journal of the American Academy of Dermatology” Tofacitinib caused significant hair regrowth in adolescents with alopecia universalis who didn't respond to other treatments.
 238 citations
,
November 2016 in “Journal of The American Academy of Dermatology”
238 citations
,
November 2016 in “Journal of The American Academy of Dermatology” Tofacitinib is effective and safe for severe hair loss, but full regrowth is less likely after 10 years of hair loss.
 139 citations
,
November 2016 in “Journal of the American Academy of Dermatology”
139 citations
,
November 2016 in “Journal of the American Academy of Dermatology” Tofacitinib helped regrow hair in most adolescents with alopecia areata, but more research is needed.
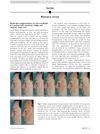 157 citations
,
December 2015 in “Journal of the American Academy of Dermatology”
157 citations
,
December 2015 in “Journal of the American Academy of Dermatology” A man with vitiligo and alopecia saw quick skin and hair improvement with ruxolitinib, but skin color gains were lost after stopping treatment.
 184 citations
,
February 2015 in “EBioMedicine”
184 citations
,
February 2015 in “EBioMedicine” A patient with Alopecia Areata had complete hair regrowth after using the drug baricitinib.
 421 citations
,
April 2012 in “The New England Journal of Medicine”
421 citations
,
April 2012 in “The New England Journal of Medicine” Alopecia Areata is an autoimmune condition causing hair loss with no cure and treatments that often don't work well.
 6 citations
,
March 2019 in “JAAD case reports”
6 citations
,
March 2019 in “JAAD case reports” A new mix of anthralin and calcipotriene might help treat severe hair loss.
 148 citations
,
December 2018 in “Journal of autoimmunity”
148 citations
,
December 2018 in “Journal of autoimmunity” Alopecia areata is an autoimmune disease causing patchy hair loss, often with other autoimmune disorders, but its exact causes are unknown.
 4 citations
,
November 2018 in “JAAD case reports”
4 citations
,
November 2018 in “JAAD case reports” Alopecia areata can sometimes appear as a straight line of hair loss instead of round patches.
 4 citations
,
July 2018 in “PubMed”
4 citations
,
July 2018 in “PubMed” Oral and topical tofacitinib can help regrow hair in people with severe alopecia areata.
May 2018 in “Journal of cosmetology & trichology” Combining platelet-rich plasma therapy with prostaglandin-F eye drops can significantly regrow hair in alopecia universalis.
110 citations
,
December 2013 in “The journal of investigative dermatology. Symposium proceedings/The Journal of investigative dermatology symposium proceedings” Alopecia areata is a genetic and immune-related hair loss condition that is often associated with other autoimmune diseases and does not typically cause permanent damage to hair follicles.













