Rapid Skin Repigmentation on Oral Ruxolitinib in a Patient with Coexistent Vitiligo and Alopecia Areata
December 2015
in “
Journal of the American Academy of Dermatology
”
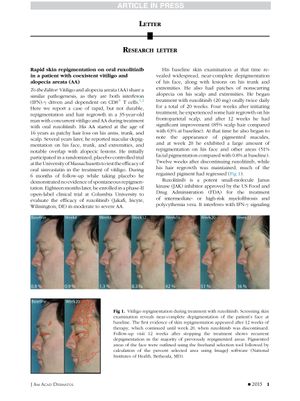
TLDR A man with vitiligo and alopecia saw quick skin and hair improvement with ruxolitinib, but skin color gains were lost after stopping treatment.
In a case study, a 35-year-old man with concurrent vitiligo and alopecia areata (AA) experienced rapid but temporary repigmentation and hair regrowth while being treated with the oral Janus kinase (JAK) inhibitor ruxolitinib. Previously, he had no repigmentation during a 6-month placebo-controlled trial for vitiligo. Later, he joined a phase-II clinical trial for ruxolitinib in treating moderate to severe AA. After starting ruxolitinib treatment (20 mg twice daily for 20 weeks), he saw hair regrowth on his scalp within 4 weeks and significant improvement (85% scalp hair) by 12 weeks. Additionally, he observed a large amount of repigmentation on his face and other areas (51% facial pigmentation) by week 20. However, 12 weeks post-treatment, while hair regrowth was maintained, much of the regained pigment had regressed. Ruxolitinib, a JAK 1 and JAK 2 inhibitor, had previously shown efficacy in reversing hair loss in AA by eliminating the interferon (IFN) signature. The patient's serum CXCL10 level, an IFN-γ-induced chemokine involved in vitiligo, was initially high but reduced after ruxolitinib treatment. This suggests that targeting the IFN-γ-CXCL10 axis might be an effective treatment strategy. Further studies are needed to assess the long-term safety and efficacy of ruxolitinib and other JAK inhibitors for vitiligo treatment.
