TLDR Older age, pre-existing skin conditions, cirrhosis, and pegylated interferon use increase the risk of skin issues during hepatitis C treatment.
This study retrospectively investigated the incidence and spectrum of dermatological adverse events (AEs) in 152 HCV-infected patients treated with interferon-alpha (IFN-α) and ribavirin from 2008 to 2012. The study found that the combination therapy, while improving sustained virological response (SVR) rates, increased the risk of cutaneous side effects, with an incidence rate of 13-34%. Factors such as previous skin conditions and the presence of cirrhosis were analyzed for their role in predisposing patients to these AEs. The study highlighted the need for careful monitoring and management of dermatological AEs in patients undergoing this treatment.
 15 citations
,
January 2014 in “Dermatology”
15 citations
,
January 2014 in “Dermatology” Some patients treated with peginterferon and ribavirin for chronic hepatitis C had mild to moderate skin reactions, but treatment did not need to be stopped.
64 citations
,
January 2009 in “Canadian Journal of Gastroenterology” Interferon and ribavirin can cause serious skin reactions and other health issues.
 41 citations
,
August 2007 in “European Journal of Gastroenterology & Hepatology”
41 citations
,
August 2007 in “European Journal of Gastroenterology & Hepatology” A woman's total hair loss from hepatitis C treatment grew back after stopping the medication.
 26 citations
,
June 2005 in “Journal of The American Academy of Dermatology”
26 citations
,
June 2005 in “Journal of The American Academy of Dermatology” Some patients receiving pegylated interferon alfa injections developed skin necrosis, requiring treatment adjustments or discontinuation.
 15 citations
,
January 2014 in “Dermatology”
15 citations
,
January 2014 in “Dermatology” Some patients treated with peginterferon and ribavirin for chronic hepatitis C had mild to moderate skin reactions, but treatment did not need to be stopped.
 1 citations
,
January 2010 in “Elsevier eBooks”
1 citations
,
January 2010 in “Elsevier eBooks” Any drug can cause skin reactions, but antibiotics, NSAIDs, and psychotropic drugs are more common, with some reactions being life-threatening.
64 citations
,
January 2009 in “Canadian Journal of Gastroenterology” Interferon and ribavirin can cause serious skin reactions and other health issues.
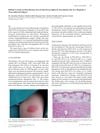 2 citations
,
January 2007 in “Acta Dermato Venereologica”
2 citations
,
January 2007 in “Acta Dermato Venereologica” A patient developed a blister at the injection site after hepatitis C treatment.
 34 citations
,
August 2002 in “British Journal of Dermatology”
34 citations
,
August 2002 in “British Journal of Dermatology” ALA-PDT is effective and safe for chronic X-ray dermatitis, providing complete or partial remission.





