Follicular and Epidermal Alterations in Patients Treated with ZD1839 (Iressa), an Inhibitor of the Epidermal Growth Factor Receptor
September 2002
in “
British journal of dermatology/British journal of dermatology, Supplement
”
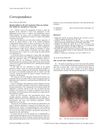
ZD1839 Iressa epidermal growth factor receptor follicular papules pustules diffuse fine scaling hair growth abnormalities superficial purulent folliculitis disordered differentiation focal parakeratosis tretinoin cream minocycline EGF receptor-targeted agents EGF receptor signalling Iressa epidermal growth factor receptor tretinoin minocycline
TLDR ZD1839 (Iressa) causes skin and hair side effects, treatable with tretinoin cream and minocycline.
Three patients treated with ZD1839 (Iressa), an inhibitor of the epidermal growth factor receptor, developed skin side effects including follicular papules, pustules, and diffuse fine scaling. Two patients also experienced hair growth abnormalities. Histological examination revealed superficial purulent folliculitis and disordered differentiation with focal parakeratosis. The follicular eruption responded well to tretinoin cream and minocycline. These cutaneous side effects were consistent with those seen with other EGF receptor-targeted agents, resulting from direct interference with EGF receptor signalling in the skin.