FDA Reports of Alopecia as an Adverse Event to Isotretinoin
July 2019
in “
Journal of Cutaneous Medicine and Surgery
”

TLDR Isotretinoin may cause hair loss, especially in women and young adults.
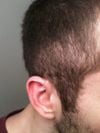
Between 1997 and 2017, the FDA received 932 reports of alopecia as an adverse event related to isotretinoin use, which accounted for 9% of all dermatological adverse event reports. The majority of these reports, 68.7%, involved women, and the most affected age group was 15- to 30-year-olds, representing 62.7% of cases where age was reported. The study suggests that isotretinoin may cause hair loss similar to telogen effluvium, where there is an arrest of the hair growth phase and defective hair anchoring. However, the study is limited by the reliance on self-reporting to the FAERS database, and the lack of information on the total number of isotretinoin prescriptions prevents the determination of the actual rate of alopecia among users. Despite these limitations, the consistent reporting over two decades indicates that physicians should be aware of the potential for hair loss in patients taking isotretinoin, and further prospective studies are needed to better understand the incidence and characteristics of this side effect.