Drug Pipeline Q2 2022: A Downturn in Approvals
August 2022
in “
Nature Biotechnology
”
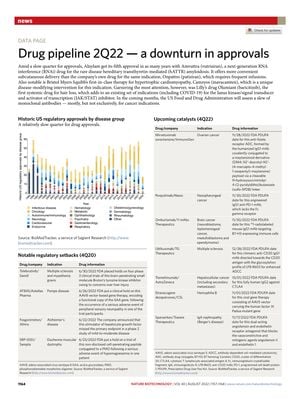
TLDR Drug approvals slowed in 2Q22, but notable drugs like Amvuttra, Camzyos, and Olumiant were approved.
In the second quarter of 2022, there was a notable slowdown in drug approvals. Despite this, Alnylam received its fifth approval in five years for Amvuttra (vutrisiran), an RNA interference drug for hereditary transthyretin-mediated amyloidosis, offering a more convenient delivery method than its predecessor. Bristol Myers Squibb introduced Camzyos (mavacamten), a first-in-class therapy for hypertrophic cardiomyopathy. Eli Lilly's Olumiant (baricitinib) gained attention as the first systemic drug for hair loss, expanding its use beyond existing indications like COVID-19. The FDA is set to review several monoclonal antibodies, primarily for cancer, in the upcoming months.



