Colorimetric Assay for Gamma-Glutamyl Transpeptidase
November 1971
in “
Clinica Chimica Acta
”
TLDR The document concludes that measuring γ-glutamyl transpeptidase activity is more accurate with a higher substrate concentration and using diluted acetic acid to stop the reaction.
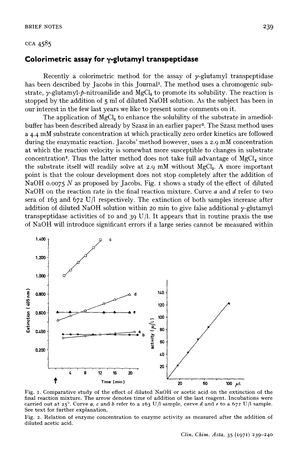
The document discusses a colorimetric method for assaying γ-glutamyl transpeptidase (GGT) activity, critiquing a method described by Jacobs that uses γ-glutamyl-p-nitroanilide as a substrate and MgCl₂ to enhance solubility. The authors argue that Jacobs' method, which uses a 2.9 mM substrate concentration, is susceptible to changes in substrate concentration and that the addition of NaOH to stop the reaction does not completely halt color development, potentially leading to significant errors in GGT activity measurements. They present an alternative method using a 4.4 mM substrate concentration and diluted acetic acid to stop the reaction, which shows no residual activity and maintains color stability for up to 16 hours at room temperature. The authors propose modifications to Jacobs' procedure, including incubation at 25°C, using bicine-buffer instead of amediol-buffer, increasing substrate concentration to 4.4 mM, and stopping the reaction with 1.1 N acetic acid. The document concludes that activity is directly proportional to the amount of serum when using diluted acetic acid to stop the reaction.



